Abstract
A Screenshot of Bacterial Biofilm Research: Remarkable Divergence Between Basic Research and Clinical Trials.
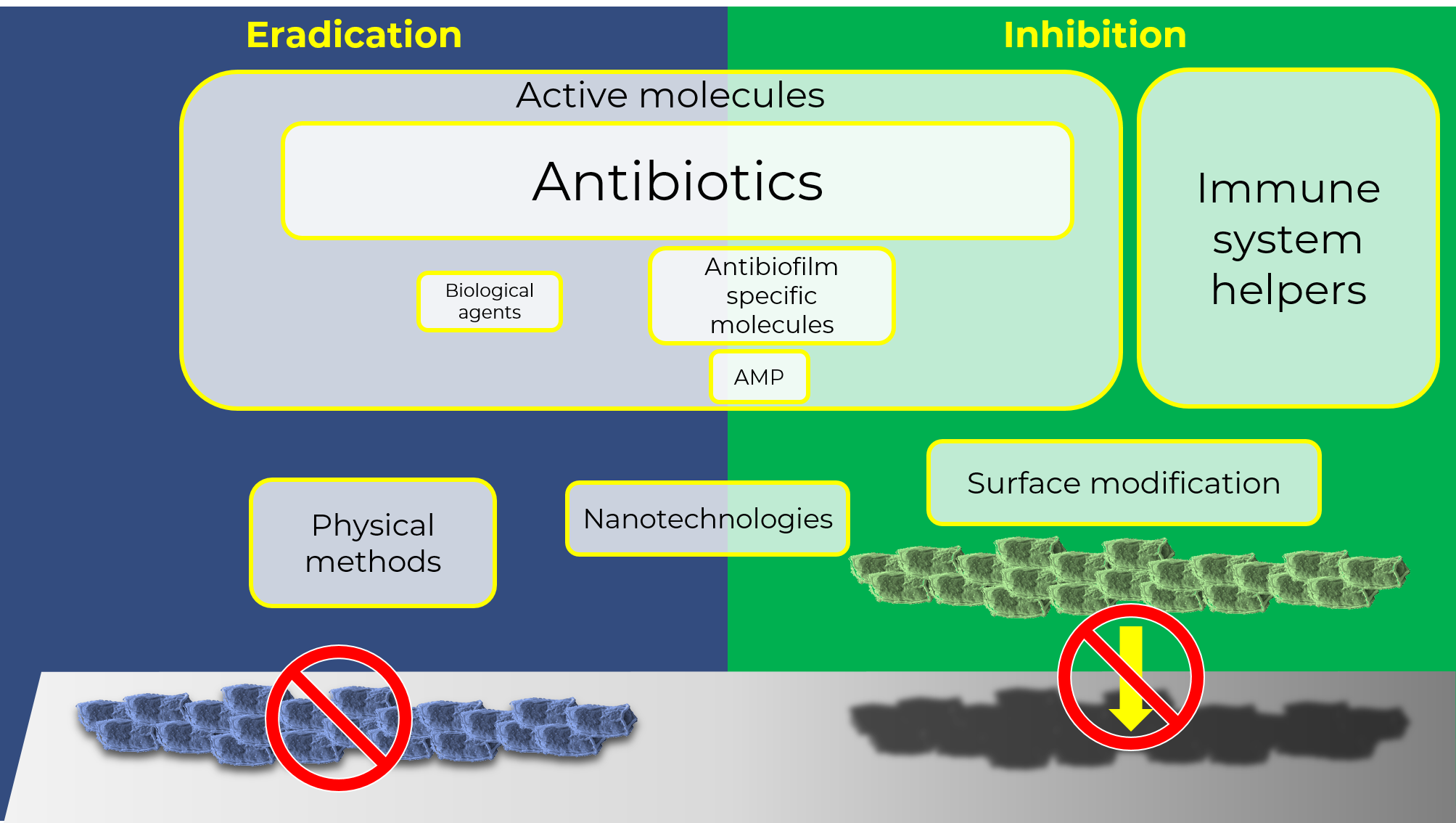
Despite the growing knowledge of biofilms as the main life condition of microbes in the past 20 years, the majority of the strategies currently employed in the clinic to face bacterial infections does not always take biofilms into account. The different adaptive strategies between free cells and biofilms and the emergence of more and more resistant strains underscore the need for innovative approaches. We tried to highlight the gap between academic research findings and clinical practice, as shown by the difference between what’s described in the scientific literature and the strategies that reach clinical trials. To shed light on recent studies on bacterial biofilms involved in health-care infections, we analyzed and described the antibacterial strategies reported in academic publications (reviews published in the last 5 years) and in clinical trials, searching two of the most used free databases (Pubmed.gov and ClinicalTrials.gov, respectively) using keywords such as “biofilm” or “bacterial infections”. We focused on works related to bacterial-associated infections, healthcare-associated infections, selecting interventional trials or reviews dedicated on preventive/therapeutic strategies. In our future posts we will try to show the disconnection between these two worlds, and we will try to assess and discuss the existing gap between basic research findings and what antibacterial strategies actually reach the clinical testing stage. Specifically, the outcomes pooled from academic research demonstrate a great variability in terms of strategy applied, while clinical studies are more focused on conventional antibacterial approaches
We utilized review articles as a proxy for basic research articles as they provide a representation of the overall research trends. We focused on pathogenic biofilms and on the strategies, researchers apply in this complex and evolving research area. We pooled the outputs based on the proposed antibacterial approaches and used the same inclusion criteria both for Pubmed and ClinicalTrials items. We will then discuss this gap and the possible limitations that prevent some of the most promising strategies from reaching the market. We identified two major problematic areas: 1) Some aspects related to the new approaches, including their toxicology, mode of action, selectivity, regulatory framework, and scale-up methods, need to be further investigated and worked out before new agents or strategies can be advanced into clinical testing; 2) Due to the intrinsic heterogeneity and complexity of biofilm structures, the current lack of universal models translates into high variability in the information obtained from each platform used to grow and analyze biofilms, especially between in vitro and in vivo assays. These culprits need to be overcome to lay the foundations for advancing new promising experimental strategies into human testing and closing the current innovation gap between basic and clinical research.