Our innovative mesoporous materials are based on silica, zirconia or titania micro and nanoparticles and possess unique physic-chemical properties which make them suitable for active pharmaceutical ingredients (API) loading, storage, protection from degradation, combination, controlled and localized release. Mesoporous materials have high surface area and large pore volume that allow hosting large and small molecules. The tailorable properties (surface charge and active groups, pore size and volume, particle size) are specifically designed for research, development and different stages of clinical investigation through the commercial manufacturing
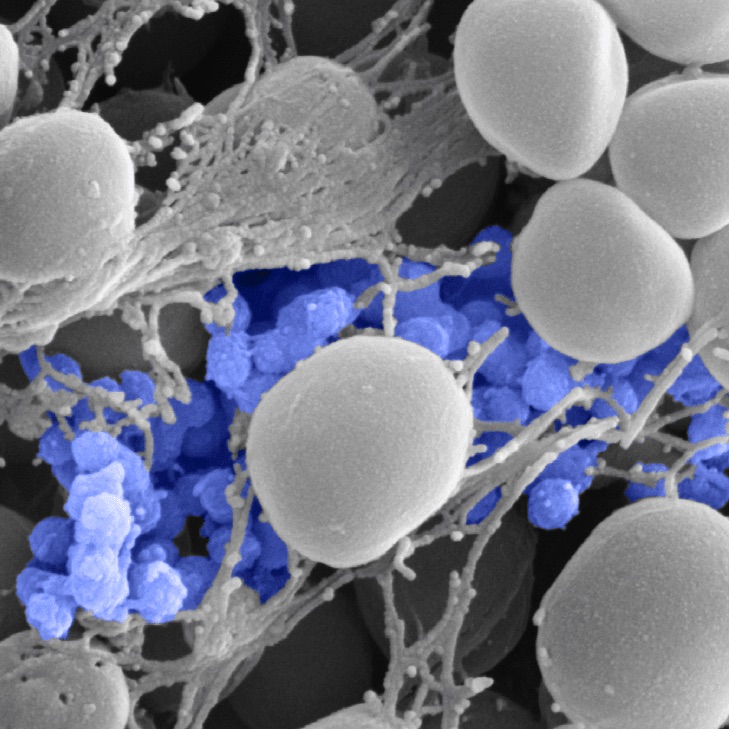
Bane
BAnE is the acrostic of Biofilm Antibiotic Enhancers. This is an example of one of our internal projects looking for business development partnerships. Every project starts with the mission definition: we dare to bane biofilm spreading, focusing for example on the major players responsible of many of the nosocomial infections. As stated by OECD in their report published in November 2018[1], “Antimicrobial resistance (AMR) is a large and growing problem with the potential for enormous health and economic consequences”. Biofilm is a complex consortium of microorganisms. The consortium is structured and surrounded by an extracellular matrix, which acts as a scaffold and as a shelter. A strategy that combines antibiotic substances with specific agents that destroy extracellular polymeric substances, is what we are working on. Aim is so to have a multipotent transporter into the biofilm, effective at the minimum dosage in order to overcome solubility and permeability issues which limit the application of many drug substances.
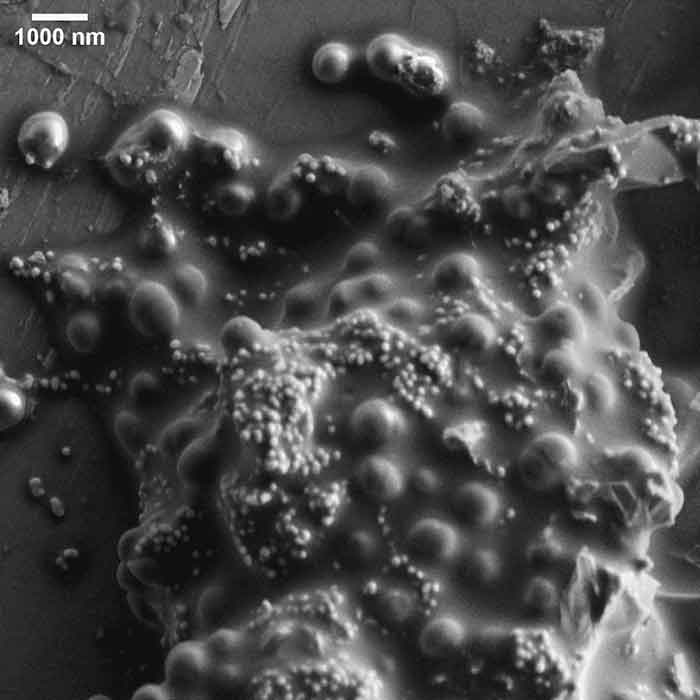
Nanocarrier
We believed there is a requirement in paradigm change on chemical pharmaceutical manufacturing. Chemical-pharmaceutical manufacturing faces many issues like:
- drug product and/or drug substance stability (cold supply chain for small molecule, large molecule and vaccines);
- handling, finishing milling and/or micronization;
- delivery of large/complex molecules;
- drug substance combinations in a unique dose form;
- biopharmaceutical classification system (salt formation, permeability and solubility, prodrugs, excipients, more doses, adverse events, therapeutic index). Our aim is to overcome these issues simplifying the supply chain and mind only the drug substance pharmacodynamic.
Il nostro scopo è superare queste problematiche semplificando la supply chain avendo sempre presente la farmacodinamica del principio attivo.
